In industrialized countries, obesity and diabetes are among the greatest health problems of this century. In fact, the rate of obesity has almost tripled worldwide in the past 50 years.1 It affects all population groups (children, adults, the elderly) without any difference in social and economic status. Obesity is a major risk factor for serious diet-related, non-communicable diseases, including type 2 diabetes. positively regulated by a peptide hormone called ghrelin.2 Thus, ghrelin is an important endocrinological target for the treatment of obesity.
The function of ghrelin
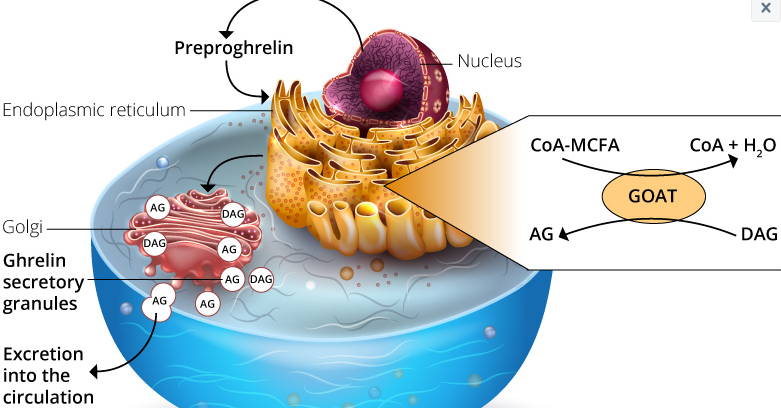
Ghrelin was discovered in 1999 by Masayasu Kojima, Kenji Kangawa and their colleagues. It is a 28 amino acid hormone that is expressed by the preproghrelin gene GHRL in enteroendocrine cells of the gastrointestinal tract. It stimulates the release of the growth hormone (GH) by binding the growth hormone secretagogue receptor (GHSR) 1a in the anterior pituitary wall.3 GHSR1a is also found in the pancreatic islets, the adrenal glands, the myocardium, the thyroid, the hypothalamus, the hippocampus and the midbrain.4 In the hypothalamus, GHSR1a is found in the arcuate nucleus (ARC) and is also co-localized in neurons that express the neuropeptide Y (Npy) and the agouti-related peptide (Agrp). These peptides are associated with food intake and satiety regulation.5 To activate GHSR, ghrelin must be acylated at position 3 by a fatty acid side chain on the serine. This post-translational modification is made possible by the ghrelin-O-acyl transferase (GOAT). This modification, mainly octanoylation and / or decanoylation, is mandatory for the effect of ghrelin on metabolism. Interestingly, the substrates (lipids) for ghrelin acylation, which are mediated by GOAT, come almost exclusively from the fats that have been ingested. These short to medium chain fatty acids must be thioesterified with the coenzyme A so that the GOAT des-acyl-ghrelin can acylate to serine-3-acyl-ghrelin.6,7 Ghrelin, modified with an octanoyl or decanoyl group, is the optimal ligand to activate GHSR1a. Whereas the role of des-acyl-ghrelin and acyl-ghrelin is still unclear. 4
The serum half-life of acyl ghrelin was found to vary between 240 minutes in humans and 30 minutes in rats. This could be because the enzymes responsible for deacylation and cleavage of ghrelin differ from species to species.8 Most studies that analyzed both des-acyl and acyl ghrelin found that most of the circulating Ghrelin is deacylated. However, a receptor for des-acyl-ghrelin has not yet been identified. Today it is assumed that all circulating ghrelin must be acylated. The presence of des-acyl ghrelin is considered an artifact of sample handling. 9
Ghrelin secretion / administration stimulates both food intake and GH secretion (in vivo and in vitro) .10 Ghrelin has been termed the “hunger hormone” for its ability to increase mammalian food intake. This designation has not been proven according to the latest research. Rather, the hormone can help prepare the body for food by regulating energy homeostasis, gastric motility, and gastric acid production. Energy homeostasis is modulated by activating orexigenic neuronal circuits. In addition, ghrelin was found to affect glucose metabolism, modulate sleep and taste, suppress the thermogenesis of brown fats, and protect against muscle loss and improve cardiovascular functions (Figure 1) .4
Ghrelin and obesity
Another important role of ghrelins is to stimulate key enzymes that promote fatty acid storage and can lead to obesity. Ghrelin is believed to act as a messenger between the body’s energy stores and the brain. This mechanism can be independent of food intake, mediated by the sympathetic nervous system. Four mechanisms are known to increase the likelihood of ghrelin-induced obesity: 10
The metabolic preference for carbohydrates instead of fat as a source of energy
Switching to a high-fat diet
Reduction of the metabolic function by lowering the resting metabolism and the heat production
Reduction of energy consumption by reducing spontaneous movement activity
By understanding these ghrelin-induced metabolic mechanisms and the compounds involved, new therapeutics can be considered. These should then target the metabolic functions of ghrelin that are still to be explained. It also helps to understand the possible causes that lead to metabolic dysfunction in obese people.10 Interestingly, no difference in weight gain or feeding behavior could be found in genetically modified mice compared to the wild type. These had both a deletion of the ghrelin gene and the GSH-R gene.10 Ghrelin knockout mice also responded to fasting and diet-related obesity like the wild type. The effect of ghrelin on the development of obesity and the associated metabolic disorders could be observed in young mice. In contrast, 18-week-old mice were resistant to diet-related obesity.12 The future of obesity research would therefore benefit from further studies on ghrelin-induced metabolic energy homeostasis. Now that we know where and how ghrelin works in some cases, a better understanding of its role in energy regulation is needed to develop new diagnostic and therapeutic approaches.